
Packing size:
1 pc/box 、 5 pcs/box
20 pcs/box 、50 pcs/box
100 pcs/box

Specimen type:
Nasopharyngeal secretion
Throat secretion
Anterior nasal secretion

Detection Time:
15-20minutes

Shelf Life:
24 months

Storage:
2-30C°

Packing Size:
1 pc/box 、 5 pcs/box
20 pcs/box 、50 pcs/box
100 pcs/box

Specimen Type:
Nasopharyngeal secretion
Throat secretion
Anterior nasal secretion

Detection Time:
15-20minutes

Shelf Life:
24 months

Storage:
2-30C°

Packing size:
1 pc/box 、 5 pcs/box
20 pcs/box 、50 pcs/box
100 pcs/box

Specimen Type:
Nasopharyngeal secretion
Throat secretion
Anterior nasal secretion

Detection Time:
15-20minutes

Shelf Life:
24 months

Storage:
2-30C°


Packing size:
1 pc/box 、 5 pcs/box 、20 pcs/box
50 pcs/box 、100 pcs/box

Storage:
2-30C°

Detection Time:
15-20minutes

Specimen type:
Nasopharyngeal secretion
Throat secretion
Anterior nasal secretion

Shelf Life:
24 months


Packing size:
1 pc/box 、 5 pcs/box 、20 pcs/box
50 pcs/box 、100 pcs/box

Storage:
2-30C°

Detection Time:
15-20minutes


Packing size:
1 pc/box 、 5 pcs/box 、20 pcs/box
50 pcs/box 、100 pcs/box

Storage:
2-30C°

Detection Time:
15-20minutes
Watch us
Watch us
iangsu Medomics Medical Technology Co.

Test design and principle
Test design and principle
SARS-CoV-2 & Influenza A/B Antigen Combo Rapid Test Kit (LFIA) uses a double antibody sandwich method to detect SARS-CoV-2 and Influenza A/B by colloidal gold immunochromatography.
When the appropriate amount of test samples treated with lysis buffer is added to the sample well of the test cassette, the sample will move forward along the test strip by capillary action. If the sample contains SARS-CoV-2 or Influenze A/B virus nucleocapsid antigen, and the concentration is higher than the limit of detection, the antigen will form immune complexes with corresponding Nucleocapsid Protein antibody labeled with colloidal gold respectively, which are captured by N line, A line, or B line. If test sample contains SARS-CoV-2 virus, forming a red N line, indicating a positive result for SARS-CoV-2. If test sample contains Influenza A virus, forming a red A line, indicating a positive result for Influenza A. If test sample contains Influenza B virus, forming a red B line, indicating a positive result for Influenza B.

Sensitivity and Specificity
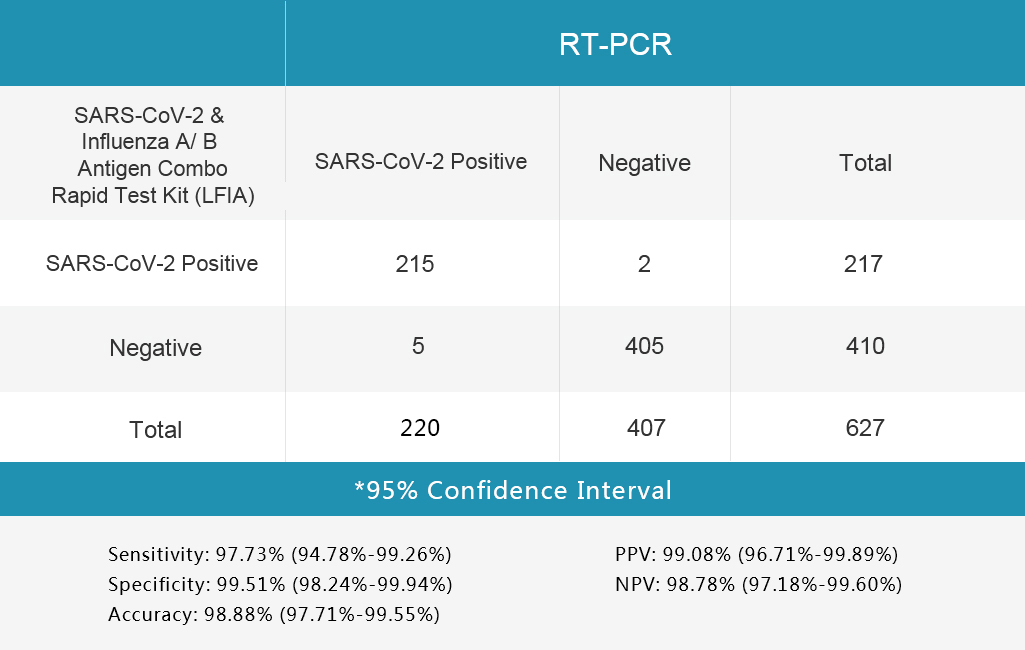
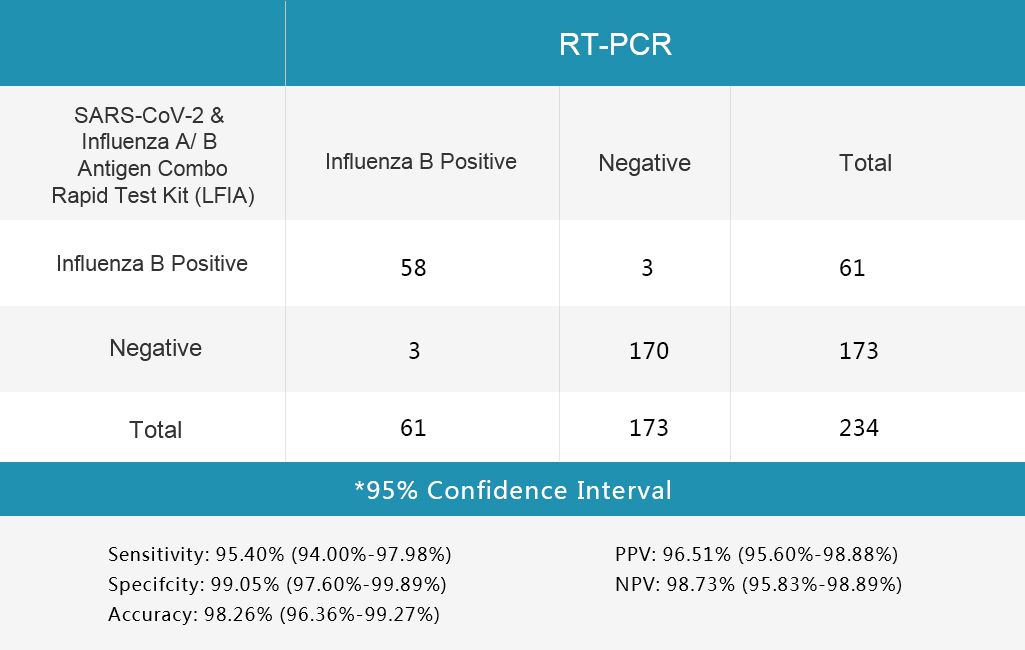
The SARS-CoV-2 & Influenza A/B Antigen Combo Rapid Test Kit (LFIA) was evaluated with different clinical samples whose status were confirmed by RT-PCR.
The results are shown in following tables.
Sensitivity and Specificity
The Medomcis SARS-CoV-2 Antigen
Test Kit (LFlA) was evaluated with different clinical
sam-ples whose status were confirmed by RT-PCR.
The results are shown in following tables.
1.Table 1 Nasopharyngeal or throat secretion collection
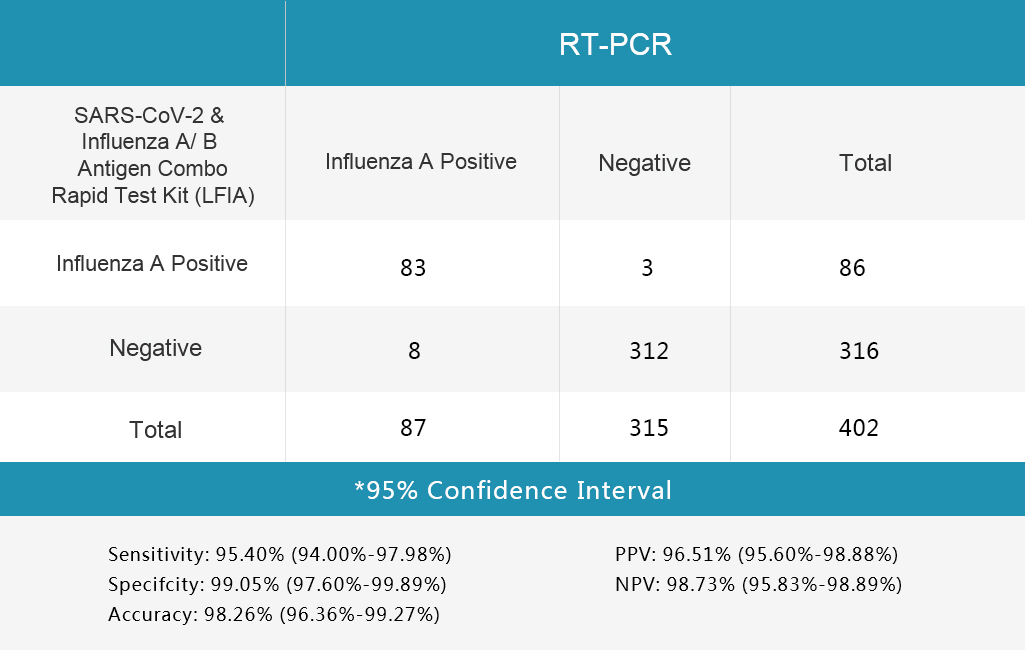
3.Table 3 Influenza A Test
2.Table 2 Anterior Nasal secretion collection
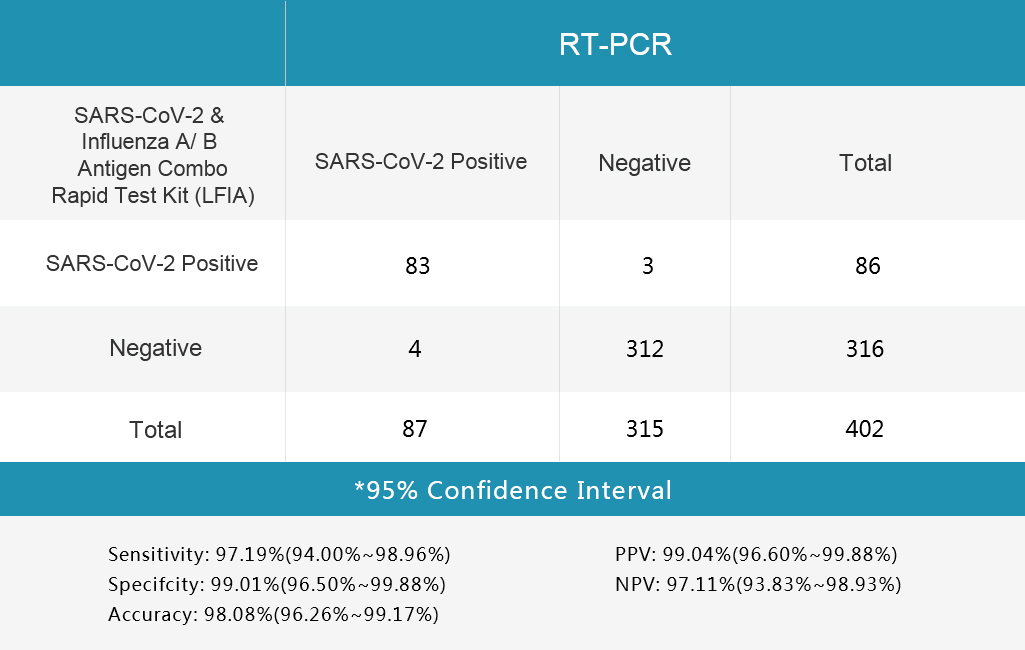
4.Table 4 Influenza B Test
Product Performance
Product Performance

FOR IN VITRO DIAGNOSTIC USE ONLY.
PLEASE READ INSTRUCTIONS CAREFULLY
BEFORE YOU PERFORM THE TEST.
FOR IN VITRO DIAGNOSTIC USE ONLY.
FOR IN VITRO DIAGNOSTIC USE ONLY.

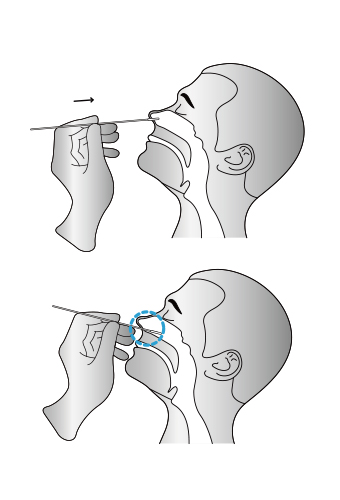
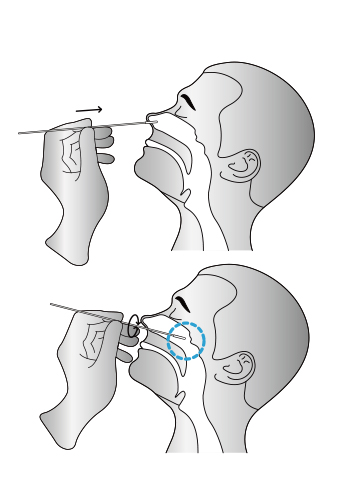
Sample Requirements
Sample Requirements
01
02
03
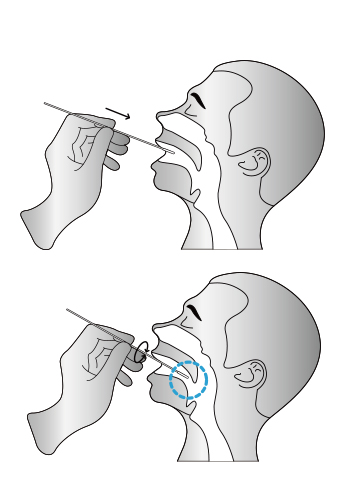
Insert the whole swab completely into the throat from the mouth, centering on the throat wall and the
reddened area of the palate tonsil, wipe both sides of the pharyngeal tonsil and posterior pharyngeal wall with moderate force. Try to avoid the tongue before taking it out.
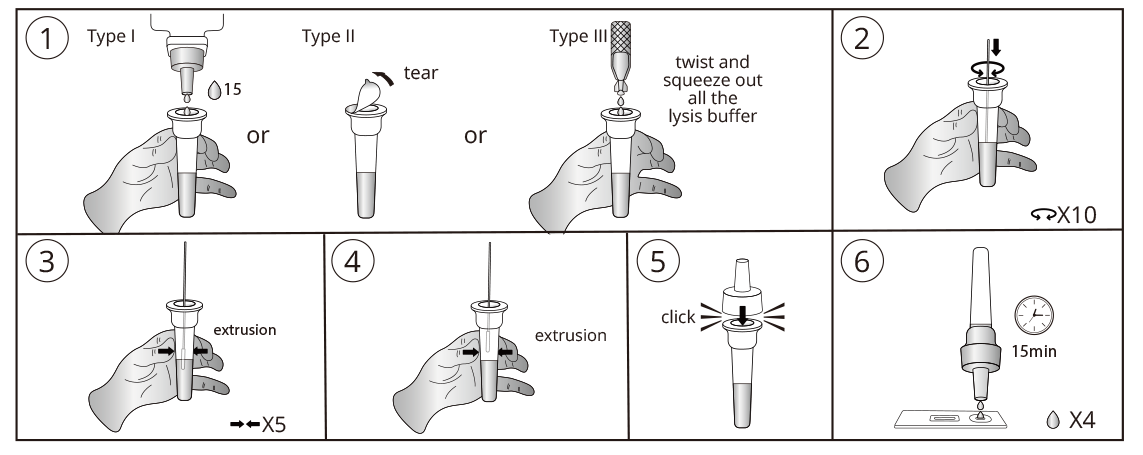
Test Procedure
Test Procedure
Do not open pouch until ready to use. Prep necessary materials: Timer | Tube rack for sampling tubes and specimens | Any necessary personal protective equipment.
1 | Sampling: vertically add 15 drops (approximately 350 μL) lysis buffer into the sampling tube from vial or open the seal of the sampling tube containing lysis buffer or twist and squeeze out all the lysis buffer into the sampling tube from capsule. Insert the swab (after collection) into the buffer. Rotate the swab against the inner tube wall 10 times and squeeze the swab from the outer tube wall 5 times to completely dissolve the sample in the buffer, the outer tube wall in order to leave the sample in the tube as much as possible.Remove and discard the swab, cover the tube with the dropper.
2 | Test procedures: Open the aluminum foil pouch, take out the test cassette and lay it on a clean flat surface, then mark the cassette with the patient ID or sample number and add 4 drops (approximately 100 μL) processed sample extract into the sample well. The result should be observed within 15-20 minutes. Results observed after 20 minutes are invalid.
DISPLAY OF THE RESULT / EXPECTED VALUE
DISPLAY OF THE RESULT / EXPECTED VALUE
“C”: Control Line
“A”: Influenza A Test Line
“B”: Influenza B Test Line
“N”: SARS-CoV-2 Test Line
“S”: Sample Well
“C”:Control Line
“A”: Infuenza A Test Line
“B”: Infuenza B Test Line
“N”: SARS-CoV-2 Test Line
“S”: Sample Well
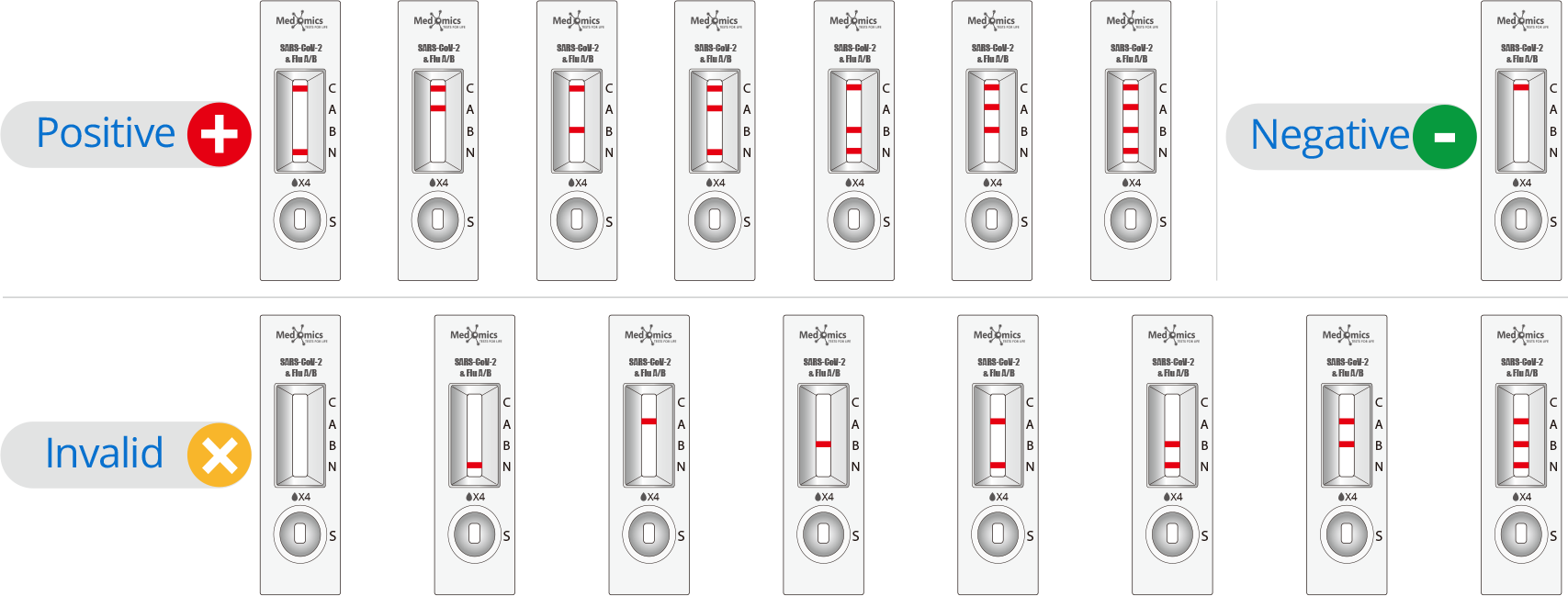
Display of Results/Expected Values
Display of Results/Expected Values
• Negative result: If only the control line (C line) appears and the test line (N line, A line and B line) is invisible, the sample does not contain SARS-CoV-2 and Influenza A/B antigen or the antigen concentration is lower than the limit of detection, then the result is negative.
• SARS-CoV-2 positive result: If the control line (C line) and the test line (N line) appear at the same time, it means that the SARS-CoV-2 has been detected and the result is positive.
• Influenza A positive result: If both the control line (C line) and the Influenza A test line (A line) appear at the same time, it means that Influenza A antigen has been detected in the sample and the result of Influenza A is positive.
• Influenza B positive result : If both the control line (C line) and the Influenza B test line (B line) appear at the same time, it means that Influenza B antigen has been detected in the sample and the result of Influenza B is positive.
• Influenza A/B positive result: If there are three lines of control line (C line), Influenza A tes line (A line), Influenza B test line (B line) shown at the same time, it means that Influenza A/B antigen have been detected in the sample.
• Invalid result: If the C line does not appear, the result is invalid and a new test must be performed again.
Note: The intensity of color that the test line area (N line/A line/B line) shows will vary according to the concentration of SARS-CoV-2 antigen, Influenza A antigen and Influenza B antigen. The result should be determined on whether the N line is formed or not, and is irrelevant to the color intensity. Therefore, any intensity of color in the test area (N line/A line/B line) should be considered positive.
Test Method Limitations
Test Method Limitations
• The accuracy of the test is dependent on the quality of the sample. Improper sampling or storage, using expired samples or repeated frozen-thawed samples can affect the test result. Test results can also be affected by temperature and humidity.
• Negative results may be caused by low concentration of SARS-CoV-2, Influenza A and Influenza B antigens in the sample and therefore cannot completely rule out the possibility of infection.
• Some medication (e.g. high concentration of over-the-counter (OTC) or prescription medication such as nasal spray) in the collected samples may interfere with the test result. Please perform the test again if the result is in doubt.
• This product is only for qualitative testing and the specific concentration of each indicator
must be measured using other quantitative methodologies.
• The results of this test are for clinical reference only and should not be the only basis for diagnosis. Results should be used in combination with clinical observations and other testing methods.
Related Products
Read more +
Related Products
Read more +
Search for more you need
Enter keywords, you can query more product information you would like to know.
Contact Information
E-mail:
Address:Building 01, Phase 6, No.71, Xinghui Road, Jiangbei New Area, Nanjing
About Us
Product Center
Jiangsu Medomics Medical Technology Co,Ltd Powered By www.300.cn 苏ICP备18065762号